Strategy Consulting
Registration & Development Strategy
Needs/Gap Assessment
Agency Strategy and Interactions
Scientific Advice
Product Lifecycle Support
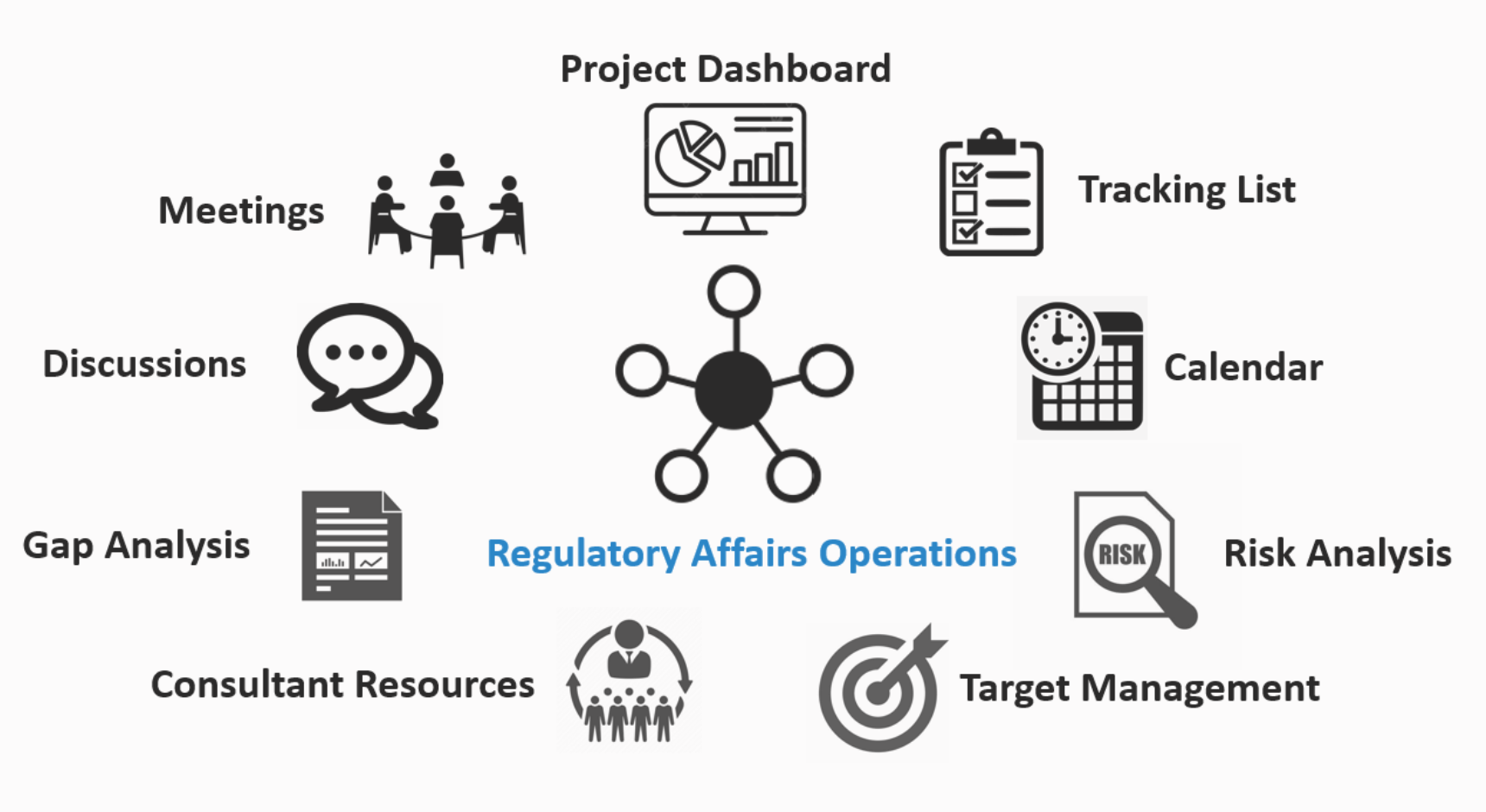
Regulatory Affairs Operations
Pre-IND IND/CTA NDA/BLA Applications
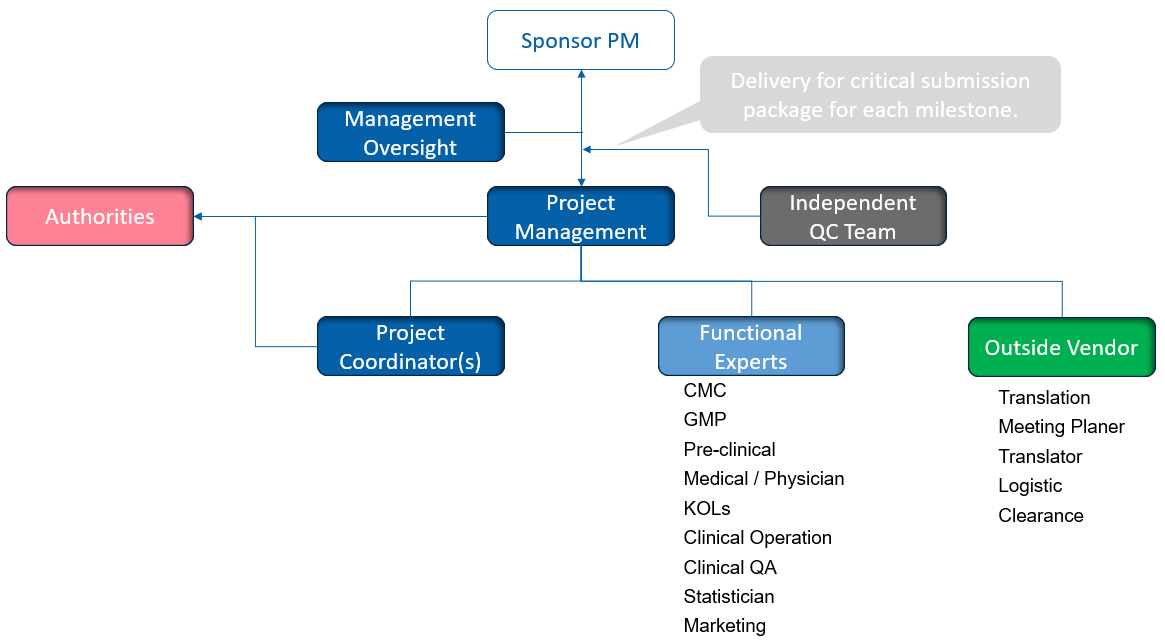
Project Management and Tracking
Lifecycle, Application & Dossier Maintenance
Authoring of all CTD sections
eCTD Publishing and Submission
Regulatory Dossiers Writing
2.3 QOS and Module 3
2.4 and 2.6 Nonclinical Overview and Summary
2.5 and 2.7 Clinical Overview and Summary
Clinical Essential Documents
Materials for Authority Interactions
Medical Device
Product Classification/Attribute Determination
Product Testing Service
IVD Regulatory Services
Authoring of Medical Device Documents
Authorities Interactions
Intelligence Service
Drug Regulatory Intelligence
Device Regulatory Intelligence
Competitor Intelligence
Interpretation of regulations and policies
Customized RI database maintenance
Other Regulatory Services
DMF preparation and filling
Regulatory SOP Supporting
GXP consultant
Regulatory training
Dossiers Translation & Proofreading